 We are pleased that Dr. Michael Fisher has joined the FTCLDF website as a regular contributor. Dr. Fisher is a retired United States Department of Agriculture (USDA) Food Safety and Inspection Services (FSIS) veterinarian, bringing decades of experience enforcing FSIS regulations during the slaughter and processing of animals for which the USDA provides inspection services. Dr. Fisher is thrilled to bring you his expertise and guidance to help you navigate regulatory compliance. His goal is for small, USDA-inspected meat processors to succeed and to understand how to best maintain compliance and reduce regulatory issues.
We are pleased that Dr. Michael Fisher has joined the FTCLDF website as a regular contributor. Dr. Fisher is a retired United States Department of Agriculture (USDA) Food Safety and Inspection Services (FSIS) veterinarian, bringing decades of experience enforcing FSIS regulations during the slaughter and processing of animals for which the USDA provides inspection services. Dr. Fisher is thrilled to bring you his expertise and guidance to help you navigate regulatory compliance. His goal is for small, USDA-inspected meat processors to succeed and to understand how to best maintain compliance and reduce regulatory issues.
This month we consider a hazard analysis for a product in the Product with Secondary Inhibitors, Not Shelf Stable processing category. What is a Product with Secondary Inhibitors, Not Shelf Stable? If you do not know, you cannot conduct a hazard analysis.
- The Code of Federal Regulations (CFR) defines “product” to include any livestock or poultry carcass, part thereof, meat by-product, meat food product, or poultry food product capable of use as human food.[1]
- It is FSIS inspection policy that a secondary Inhibitor is an ingredient that inhibits bacterial growth.[2]
- It is FSIS inspection policy that a product is not shelf stable if it is perishable and cannot be safely stored at room temperature.[3]
What separates a not shelf stable product with secondary inhibitors from raw ground and raw not ground product is the presence of a secondary inhibitor. What separates a not shelf stable product with secondary inhibitors from all other products is the application of heat, a shelf stable status, or both.
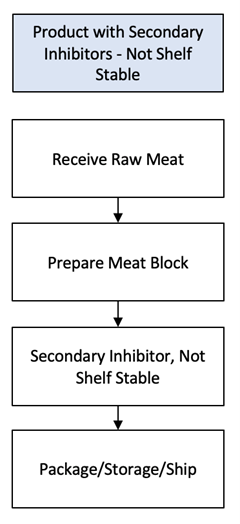
Now that we have identified a not shelf stable product with secondary inhibitors, we need a 9 CFR 417.2(a)(2) flow chart describing the process steps and product flow for preparing the product. The diagram to the left is such a flow chart. Remember, a process is a series of steps conducted to an end, and a step is a unique activity that can encompass multiple actions. Our end is a not shelf stable product with secondary inhibitors.
Grounds, facilities, equipment, utensils, chemicals, non-meat ingredients, and employees are potential sources of food safety hazards and common to all process steps. Maintaining 9 CFR 416 compliance prevents insanitary conditions and ensures that product is not adulterated by grounds, facilities, equipment, utensils, chemicals, non-meat ingredients, and employees. Therefore, a 9 CFR 417.1 preventive measure is not required to control a potential food safety hazard originating from grounds, facilities, equipment, utensils, chemicals, non-meat ingredients, and employees because it is already controlled by another regulatory performance standard. So, our hazard analysis makes no determination that a food safety hazard is reasonably likely to occur due to grounds, facilities, equipment, utensils, and employees during the Product with Secondary Inhibitors, Not Shelf Stable process.
Receive Raw Meat: Raw meat or poultry are potential sources of food safety hazards. A federally inspected, not shelf stable product with secondary inhibitors must be prepared from federally inspected products. 9 CFR 318.1(a) and 9 CFR 381.145(a) require that such products be “prepared only in an official establishment and previously inspected and passed by a Program employee” to ensure they are not adulterated. The mark of inspection means that FSIS verified that the producing establishment followed the Hazard Analysis Critical Control Point (HACCP) process it determined is necessary to produce safe product.[4] USDA’s FSIS Office of Investigation Enforcement and Audit (OIEA) Compliance and Investigations Division conducts surveillance of all products in commerce to assure that such products are not adulterated. A prudent establishment that maintains 9 CFR 318.1(a) and 9 CFR 381.145(a) and 9 CFR 416 compliance is sufficiently assured that product received is not adulterated. Therefore, our hazard analysis makes no determination that a food safety hazard is reasonably likely to occur during this process step.
Prepare Meat Block: Preparing the meat block can involve cutting, grinding, mixing, injecting, or stuffing. I choose to capture these activities within a single process step. An official establishment may/may not choose the same.
Not shelf stable products with secondary inhibitors contain two or more ingredients, one of which is a secondary inhibitor. Ingredients are potential sources of food safety hazards. 9 CFR 318.6(a) requires that ingredients not result in the product being adulterated. Non-meat and -poultry ingredients are regulated by the Food and Drug Administration. FSIS accepts a guaranty by the ingredient supplier that the ingredient is safe for the intended use within the meaning of the Federal Food, Drug, and Cosmetic Act. A prudent establishment maintains such guaranty and 9 CFR 318.6(a) and 9 CFR 416 compliance. No food safety hazard reasonably likely to occur carries over from the previous process step. Therefore, our hazard analysis makes no determination that a food safety hazard is reasonably likely to occur during this process step.
Secondary Inhibitor, Not Shelf Stable: There are two methods to transform the meat block into a not shelf stable product using a secondary inhibitor: the dry method and the wet method. I choose to capture these activities within a single process step. An official establishment may/may not choose the same.
Both methods use salts, alone or in combination, and other ingredients. Historically, four salts are used: sodium chloride (i.e., NaCl) potassium nitrate (i.e., KNO3), sodium nitrate (i.e., NaNO3), and sodium nitrite (i.e., NaNO2). Other ingredients might include sugar and seasonings. The salt(s) decreases the amount of water in the meat block by exchanging the cellular water in the meat block in part for the salts and other ingredients, which reduces water activity, which inhibits growth of bacteria and prevents spoilage. Sodium chloride (i.e., table salt) is most effective at reducing water activity because it does not undergo chemical change during the process. Potassium nitrate (i.e., saltpeter), sodium nitrate, or sodium nitrite undergo chemical change and produce an additional mechanism of action, as bacteria remove one oxygen atom from the nitrate moiety (i.e., NO3) producing a nitrite moiety (i.e., NO2), which degrades into nitric oxide (i.e., NO), which has cytotoxic effects against bacteria.
With the dry method the meat block is either packed in the salt(s), or the salt(s) are rubbed into the surface of the meat block. The process is continued and repeated for weeks to months, over which time the amount of water in the meat block decreases and the nitrogen compounds produce their antimicrobial effect. Prosciutto is an example of a dry method product.
With the wet method the salt(s) are dissolved in water to create a brine (i.e., salt and water) or a pickle (i.e., brine plus additional ingredients), which is distributed throughout the meat block by submersion or injection. With submersion the meat block is typically skinned and submersed in a brine or pickle solution. The salt(s) diffuses into the meat block from the outside in. With injection the brine or pickle solution is placed directly into the meat, either by injecting the solution with needles, or pumping the solution into the meat block via the arteries. The salt(s) diffuses into the meat block from the inside out.
A prudent establishment maintains 9 CFR 416 compliance. No food safety hazard reasonably likely to occur carries over from the previous process step. No new ingredients are introduced at this process step. Therefore, our hazard analysis makes no determination that a food safety hazard is reasonably likely to occur during this process step.
Package/Storage/Ship: Packaging, storaging, and shipping are activities common to all processing categories, regardless of the product prepared. I choose to capture them within a single process step. An official establishment may/may not choose the same.
9 CFR 317.24 or 9 CFR 381.144 and 9 CFR 325.1(c) or 9 CFR 381,190(c) require that packaging materials and transport methods, respectfully, not result in product being adulterated. A prudent establishment maintains 9 CFR 317.24 or 9 CFR 381.144 and 9 CFR 325.1(c) or 9 CFR 381,190(c) and 9 CFR 416 compliance. No food safety hazard reasonably likely to occur carries over from the previous process step. Therefore, our hazard analysis makes no determination that a food safety hazard is reasonably likely to occur during this process step.
Hazard Analysis Complete! Surprised? To be expected. In 1997, FSIS published generic hazard analyses for each 9 CFR 417.2(b)(1) processing category and each generic hazard analysis identified at least one critical control point. The result was the false understanding that each 9 CFR 417.2(a) hazard analysis must identify at least one food safety hazard reasonably likely to occur. This false understanding persists today. The above hazard analysis refutes that false understanding.
Key to conducting any hazard analysis is the realization that a hazard analysis rests on a foundation of sanitation. Absent that foundation, conducting a hazard analysis is a waste of time because without sanitation, product that is not adulterated cannot be produced. The above hazard analysis is moot if the establishment fails to start the process with meat or poultry that is adulterated and fails to comply with all applicable sanitation requirements.
Footnotes
[1] 9 CFR 301.2 and 9 CFR 381.1
[2] USDA, FSIS. (2019) FSIS Product Categorization (Guideline ID: FSIS-GD-2019-0010). https://www.fsis.usda.gov/guidelines/2019-0010
[3] USDA, FSIS. (2019) What does “shelf stable” mean? (Knowledge Article). https://ask.usda.gov
[4] Paul Kiecker, Acting FSIS Administrator (personal communication, April 12, 2018)
More
As always, if you have a question, please use the Contact Us link and ask.
Did you miss Dr. Fisher’s previous posts?
YOUR FUND AT WORK
Services provided by FTCLDF go beyond legal representation for members in court cases.
Educational and policy work also provide an avenue for FTCLDF to build grassroots activism to create the most favorable regulatory climate possible. In addition to advising on bill language, FTCLDF supports favorable legislation via action alerts and social media outreach.
You can protect access to real foods from small farms by becoming a member or donating today.