 We are pleased that Dr. Michael Fisher has joined the FTCLDF website as a regular contributor. Dr. Fisher is a retired United States Department of Agriculture (USDA) Food Safety and Inspection Services (FSIS) veterinarian, bringing decades of experience enforcing FSIS regulations during the slaughter and processing of animals for which the USDA provides inspection services. Dr. Fisher is thrilled to bring you his expertise and guidance to help you navigate regulatory compliance. His goal is for small, USDA-inspected meat processors to succeed and to understand how to best maintain compliance and reduce regulatory issues.
We are pleased that Dr. Michael Fisher has joined the FTCLDF website as a regular contributor. Dr. Fisher is a retired United States Department of Agriculture (USDA) Food Safety and Inspection Services (FSIS) veterinarian, bringing decades of experience enforcing FSIS regulations during the slaughter and processing of animals for which the USDA provides inspection services. Dr. Fisher is thrilled to bring you his expertise and guidance to help you navigate regulatory compliance. His goal is for small, USDA-inspected meat processors to succeed and to understand how to best maintain compliance and reduce regulatory issues.
Over the last four months, we discussed Hazard Analysis and Critical Control Point (HACCP) terms, hazard analysis requirements, flowchart, and food safety hazards. Let’s put those discussions to work and conduct a 9 Code of Federal Regulations (CFR) 417.2(a) hazard analysis for livestock and poultry slaughter.
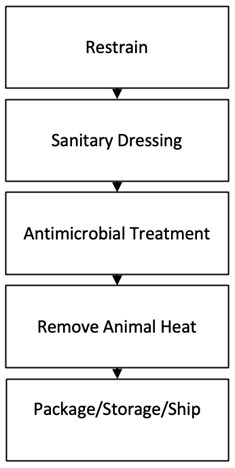 We start with the 9 CFR 417.2(a)(2) flow chart describing the process steps and product flow. Remember, a process is a series of steps conducted to an end, and a step is a unique activity that can encompass multiple actions. The end for a slaughter process is the conversion of a live animal into a wholesome carcass and parts. The first four process steps in the flowchart on the left achieve that end. The final step in the flowchart on the left encompasses multiple actions common to all processes. Prior activities such as live animal receiving are prior to, not part of, the slaughter process. Establishments often break sanitary dressing down into multiple actions: stunning, bleeding, hide/feather removal, evisceration, etc. If the additional detail aids in your analysis, then do so, but 9 CFR 417.2(a)(2) does not require that level of detail.
We start with the 9 CFR 417.2(a)(2) flow chart describing the process steps and product flow. Remember, a process is a series of steps conducted to an end, and a step is a unique activity that can encompass multiple actions. The end for a slaughter process is the conversion of a live animal into a wholesome carcass and parts. The first four process steps in the flowchart on the left achieve that end. The final step in the flowchart on the left encompasses multiple actions common to all processes. Prior activities such as live animal receiving are prior to, not part of, the slaughter process. Establishments often break sanitary dressing down into multiple actions: stunning, bleeding, hide/feather removal, evisceration, etc. If the additional detail aids in your analysis, then do so, but 9 CFR 417.2(a)(2) does not require that level of detail.
Next, we determine the food safety hazard reasonably likely to occur in the production process and identify the preventive measures the establishment can apply to control those food safety hazards, starting with the first process step.
Restrain: Feces and ingesta are present within the gastrointestinal tract and on the exterior surfaces of every animal placed in a restraining device. A prudent establishment has good reason to believe that human pathogens (i.e., biological food safety hazards) may reside in these feces and ingesta [61 FR 38806]. So, our hazard analysis determines that a biological food safety hazard is reasonably likely to occur during the Restrain process step because a biological food safety hazard is introduced into the slaughter process with the introduction of the animal during the Restrain process step. A review of the December 2020 article on Food Safety Hazards will show that a prudent establishment has no good reason to believe that a chemical or physical food safety hazard is introduced into the slaughter process with the animal during the Restrain process step. So, our hazard analysis makes no determination that a chemical or physical food safety hazard is reasonably likely to occur during the Restrain process step.
Sanitary Dressing: Fecal material is a vehicle for human pathogens [FR 62 63254]. Feces and ingesta introduced during the Restrain process step carry over into sanitary dressing. A prudent establishment has good reason to believe that the biological food safety hazard reasonably likely to occur during the Restrain process step also carries over into sanitary dressing. So, our hazard analysis determines that a biological food safety hazard is reasonably likely to occur during the Sanitary Dressing process step because a biological food safety hazard introduced during the Restrain process step carries over and is not controlled by a preventive measure during the Sanitary Dressing process step. A prudent establishment has no good reason to believe that a chemical or physical food safety hazard carries over into sanitary dressing.
Antimicrobial Treatment: Sanitary dressing eliminates visible feces and ingesta, but does not prevent, eliminate, or reduce to acceptable levels invisible human pathogens. A prudent establishment has good reason to believe that the invisible human pathogens (i.e., biological food safety hazards) introduced during the Restrain process step and carried over into the Sanitary Dressing process step, carries over into the Antimicrobial Treatment process step. So, our hazard analysis determines that a biological food safety hazard is reasonably likely to occur during the Antimicrobial Treatment process step because a biological food safety hazard introduced during the Restrain process step carried over into the Sanitary Dressing and Antimicrobial Treatment process steps. A prudent establishment has no good reason to believe that a chemical or physical food safety hazard carries over into the Antimicrobial Treatment process step.
The antimicrobial treatment is a chemical means that can be used to control the biological food safety hazard reasonably likely to occur introduced during the Restrain process step and carried over into the Sanitary Dressing and Antimicrobial Treatment process steps.
Remove Animal Heat: The 9 CFR 417.2(a)(1) requirement is “determine food safety hazards reasonably likely to occur.” It is not “determine the food safety hazards NOT reasonably likely to occur,” or “determine non-food safety hazards reasonably likely to occur.” A food safety hazard determined to be reasonably likely to occur entered the process with the Restrain process step and carried over into subsequent steps until controlled in the Antimicrobial Treatment process step. The food safety hazard does not carry over into the Remove Animal Heat step. It was controlled in the previous process step. Therefore, a food safety hazard reasonably likely to occur does not exist in the Remove Animal Heat step.
Removing Animal Heat is important. If you do not remove the heat, the meat will spoil. Spoiled meat is adulterated. It is unfit for use as human food, but not unsafe for use as human food. Spoiled meat is considered a hazard requiring a control, but it is not a food safety hazard requiring a preventive measure. Removing the animal heat is a 9 CFR 416.4(d) sanitation requirement, not a 9 CFR 417.2 requirement.
Package/Storage/Ship: The antimicrobial treatment controlled the previously identified biological food safety hazard reasonably likely to occur; therefore, a prudent establishment has no good reason to believe that either a biological food safety hazard or a chemical or physical food safety hazard carries over into the Package/Storage/Ship process step.
We identified and dealt with the food safety hazard originating from the live animal. What about food safety hazard originating from insanitary facilities, equipment, utensils, and employees? 9 CFR 416 requires official establishments to prevent insanitary conditions and ensure that product is not adulterated. A prudent establishment would not implement 9 CFR 417.1 preventive measures to control sanitary conditions controlled by other regulatory performance standards.
Hazard Analysis Complete!
You will find many formats for a hazard analysis. Choose one that works for you. You will find hazard analysis examples that contain significantly more information. Your hazard analysis should contain as much information as you need it to contain; not as much information as the FSIS inspector wants it to contain. More is not always better, or necessary. Keep it simple.
More
As always, if you have a question, please use the Contact Us link and ask.
Did you miss Dr. Fisher’s previous posts?
YOUR FUND AT WORK
Services provided by FTCLDF go beyond legal representation for members in court cases.
Educational and policy work also provide an avenue for FTCLDF to build grassroots activism to create the most favorable regulatory climate possible. In addition to advising on bill language, FTCLDF supports favorable legislation via action alerts and social media outreach.
You can protect access to real foods from small farms by becoming a member or donating today.